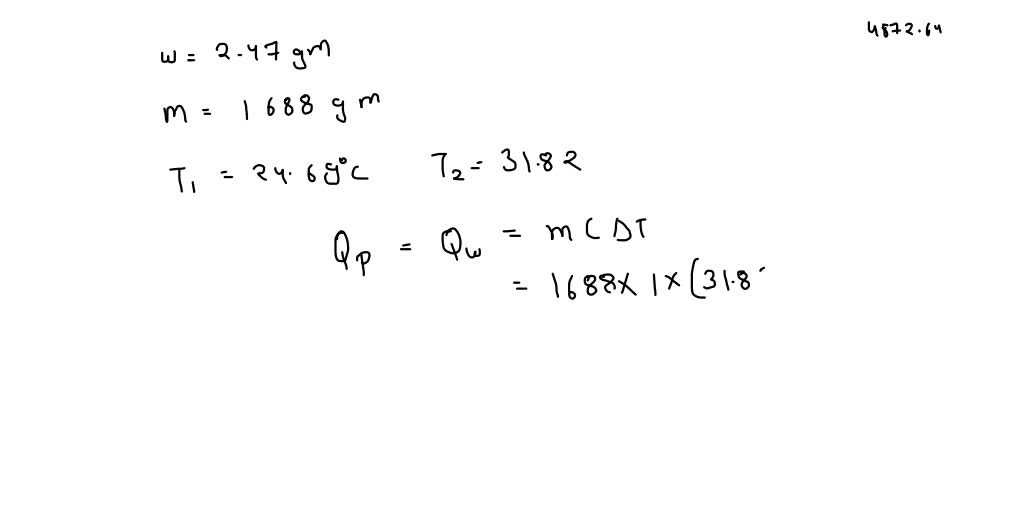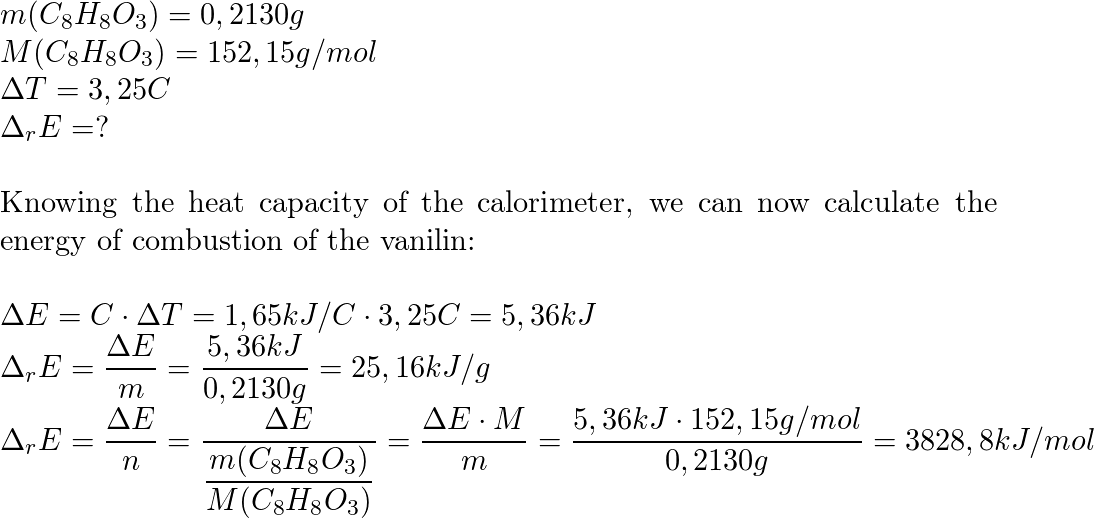Calculate the energy released by 1 g of natural uranium assuming 200 meV is released in each fission event and that the fissionable isotope ^23U has an abundance of 0.7

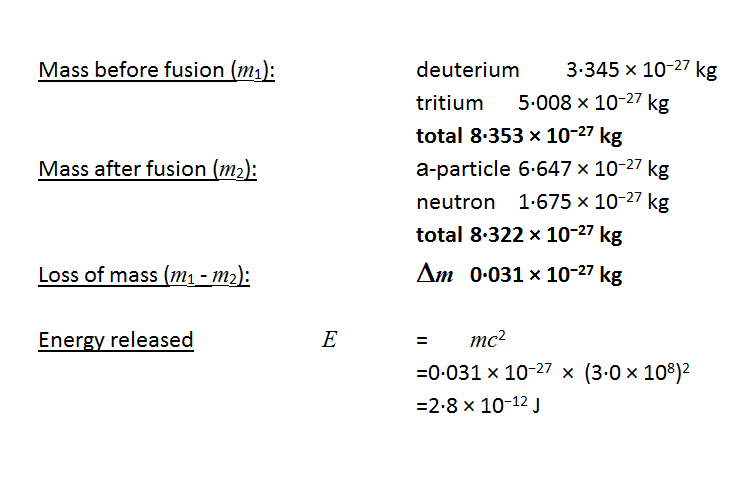
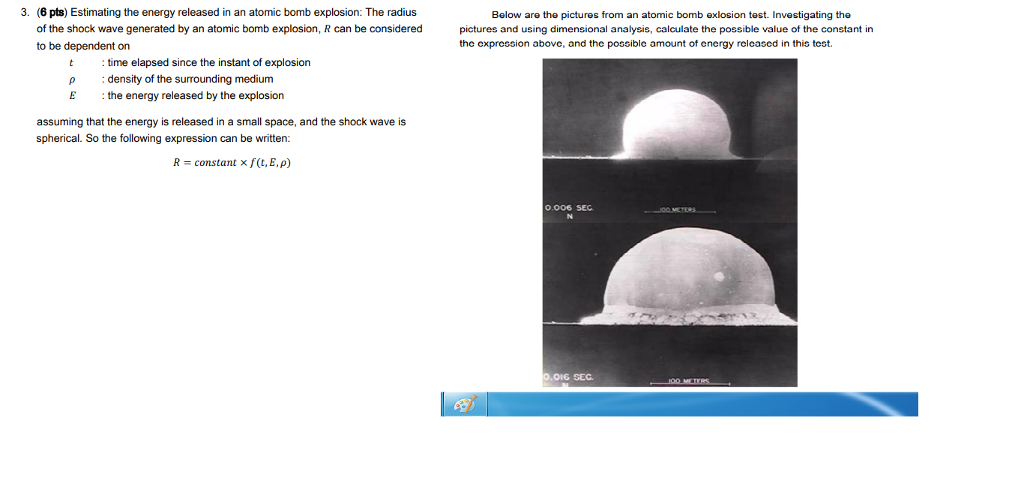
An explosion of atomic bomb releases an energy of 7.6xx10^(13)J. If 200 MeV energy is released on fission of one .^(235)U atom calculate (i) the number of uranium atoms undergoing fission. (ii)

Calculate the energy released by 1 g of natural uranium assuming 200 meV is released in each fission event and that the fissionable isotope ^23U has an abundance of 0.7
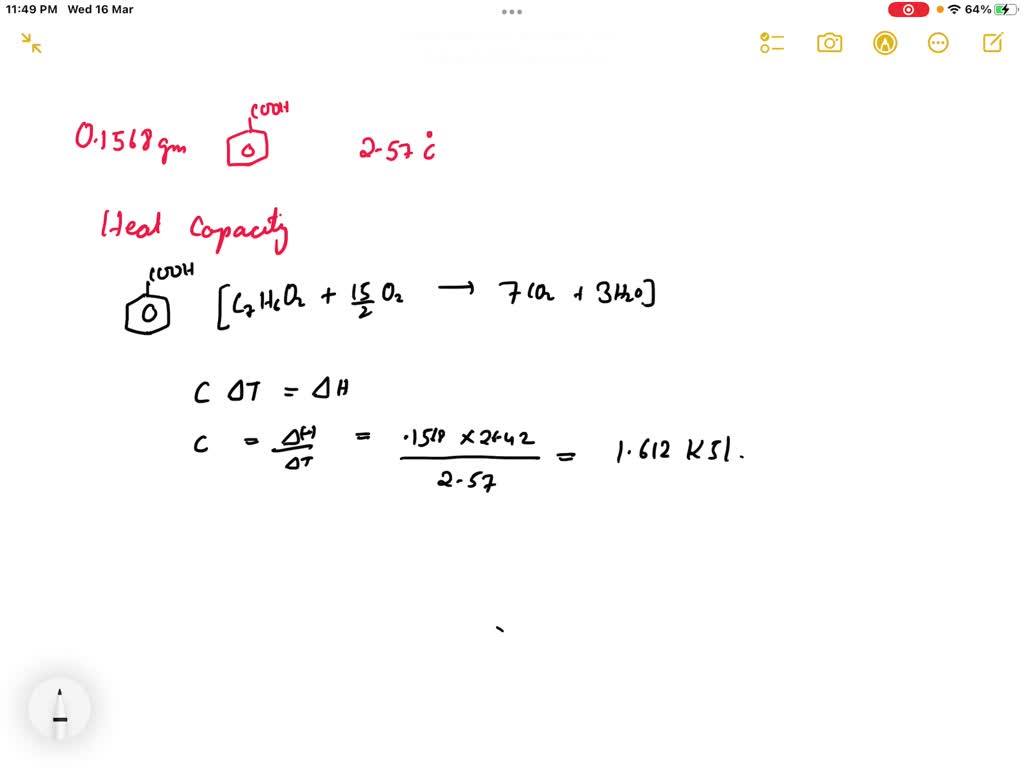
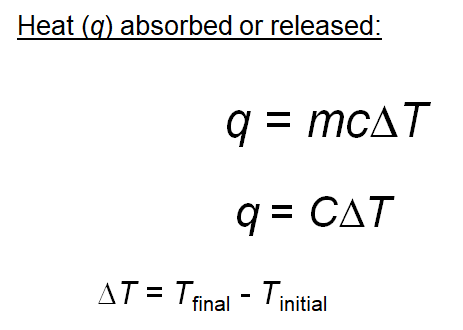
SOLVED: The combustion of 0.1568 g benzoic acid increases the temperature of a bomb calorimeter by 2.57°C. Calculate the heat capacity of this calorimeter. (The energy released by combustion of benzoic acid

An explosion of atomic bomb releases 7.6 × 10^13J energy. If 200 MeV energy is released on fission of one ^235U atom, then the number of uranium atoms undergoing fission and the
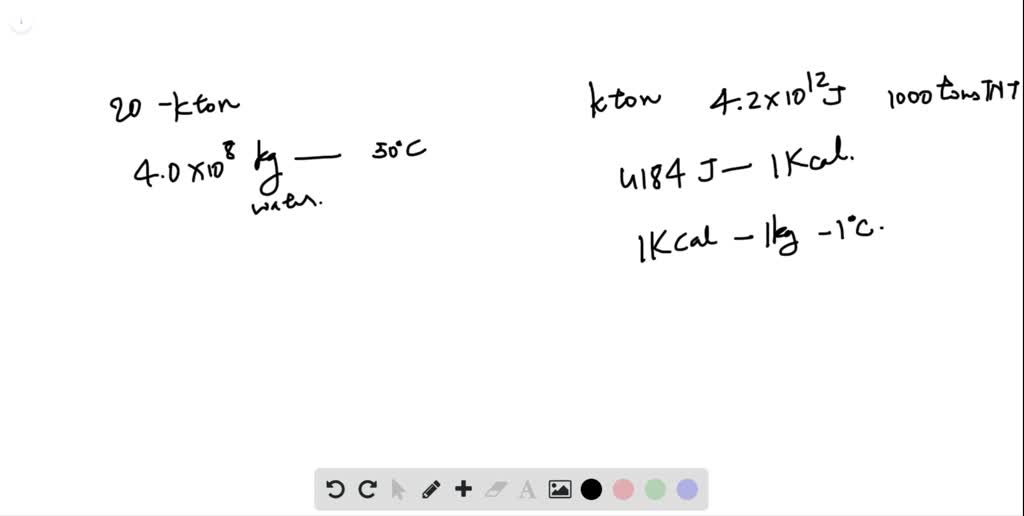
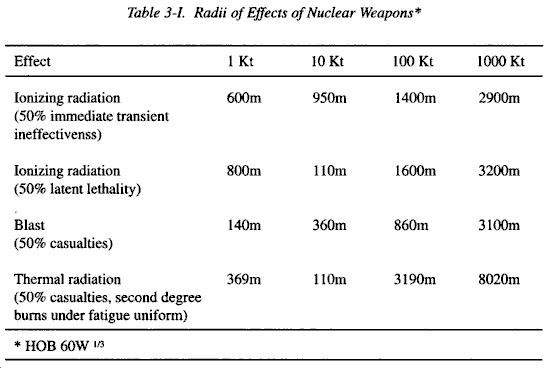
SOLVED:The kiloton, which is used to measure the energy released in an atomic explosion, is equal to 4.2 ×10^12 J (approximately the energy released in the explosion of 1000 tons of TNT).